
Commentaries | May 25,2024
Apr 29 , 2023
By Cristina Donini , Doreen Akiyo Yomoah
Over the past three years, the COVID-19 pandemic has dominated headlines and spurred scientific research, with experts around the world focusing resources and any potentially useful technology on the problem.
While the spotlight on COVID-19 has dimmed slightly, it remains a high global priority, sometimes to the detriment of infectious diseases linked to poverty and primarily affecting the Global South. For example, Malaria killed an estimated 619,000 people – most children in Sub-Saharan Africa – in 2021, when there were 247 million cases worldwide.
Malaria is an entirely preventable and treatable disease, and researchers have made great strides on both fronts. In March, for example, the World Health Organization (WTO) recommended two new dual-ingredient insecticide-treated bed nets to protect against malaria-transmitting Anopheles mosquitoes, one with a more lethal cocktail of insecticides and the other able to disrupt mosquito growth and reproduction.
Cost-effective antimalarial medicines are another important tool. In 2021, seasonal malaria chemoprevention was administered to around 45 million children aged three months to five years, who received monthly doses of therapeutic drugs at less than four dollars per person. The recent news of a groundbreaking vaccine, GSK's Mosquirix (also known as RTSS), offers some hope, although the cost is still relatively high, at around 40 dollars a child for the first year.
Despite these efforts, malaria continues to pose a threat to public health.
Even after an investment of 26 billion dollars to tackle the disease in Sub-Saharan Africa, the number of cases increased slightly between 2000 and 2019 (although the number of deaths decreased). New prevention measures – tailored to children, in particular – are needed. Further innovation should take a page from the pandemic playbook: one benefit of the flood of COVID-19 research is that it demonstrated the enormous potential of monoclonal antibodies.
These drugs are laboratory-made copies of the proteins that a person's immune system produces to attack specific foreign invaders. Historically, monoclonal antibodies have been a powerful weapon against cancer and autoimmune diseases such as rheumatoid arthritis and lupus. While not often used as a prophylactic, the deployment of monoclonal antibodies to prevent COVID-19 and the respiratory syncytial virus has shown great promise.
Moreover, their exquisite selectivity enables them to discern between closely related molecular targets, resulting in fewer off-target effects. This makes it a medication with an appropriate safety profile for children (as well as other at-risk populations).
A research group at the United States National Institutes of Health, led by Robert Seder, has identified two antibodies against CSP-1, the malaria parasite's protein, to invade liver cells where it first establishes infection. Blocking CSP-1 should thus prevent infection. The more advanced of the two antibodies, L9LS, is currently being tested for its safety and efficacy in children in Mali and Kenya.
The Mali study assesses its success in a setting of seasonal malaria, whereas the Kenya study focuses on an area where year-round infection is possible.
Monoclonal antibodies could be a game-changer for preventing malaria and advancing the long-sought goal of eradication.
The current generation of antimalarial antibodies has been modified so that a single dose can protect a child for at least three months – possibly longer. The clinical trials will determine the extent and exact duration of protection, and provide useful guidance on how much improvement is needed to achieve a dosage that can be injected once per year.
Although antibodies have a reputation for being expensive, with those used to treat cancer priced at over 20,000 dollars a month in Europe and the US, increasing the potency of this cutting-edge treatment could significantly decrease costs. Some believe an injection as small as one millilitre of the antibody drug being trialled in Mali and Kenya could protect children at the cost of only five to 10 dollars per person.
Demand for monoclonal antibodies primarily comes from high-income countries; Africa accounts for only one percent of global sales. This disparity highlights the need to work with national regulatory agencies to ensure that submitted product data adequately address public health concerns and, in the longer term, involve affected countries in producing these biologics.
Although manufacturing antibodies is a complex and highly regulated process, investing in the technology now would be a boon for developing economies burdened by endemic malaria.
Monoclonal antibodies may be the new frontier in the fight against malaria, but getting the word out won't be easy: stakeholders from government, academia, and industry must come together to coordinate advocacy efforts and raise awareness. (The same groups should encourage the development of these biologics for all infectious diseases.)
We are embarking on a long road: the first generation of antimalarial antibodies will not be deployed until 2027. They offer tremendous promise as one of many weapons to fight this child killer, alongside bed nets, medicines, and emerging vaccines.
The clinical trials will tell us whether this potential can be realized, but we would be wise to begin preparing for success now.
PUBLISHED ON
Apr 29,2023 [ VOL
24 , NO
1200]

Commentaries | May 25,2024

Fortune News | Oct 31,2020

Fortune News | Mar 25,2023

Radar | Jun 01,2024

Radar | Oct 24,2020

Commentaries | Jun 08,2024

Fortune News | Oct 22,2022

Fortune News | Jul 20,2019
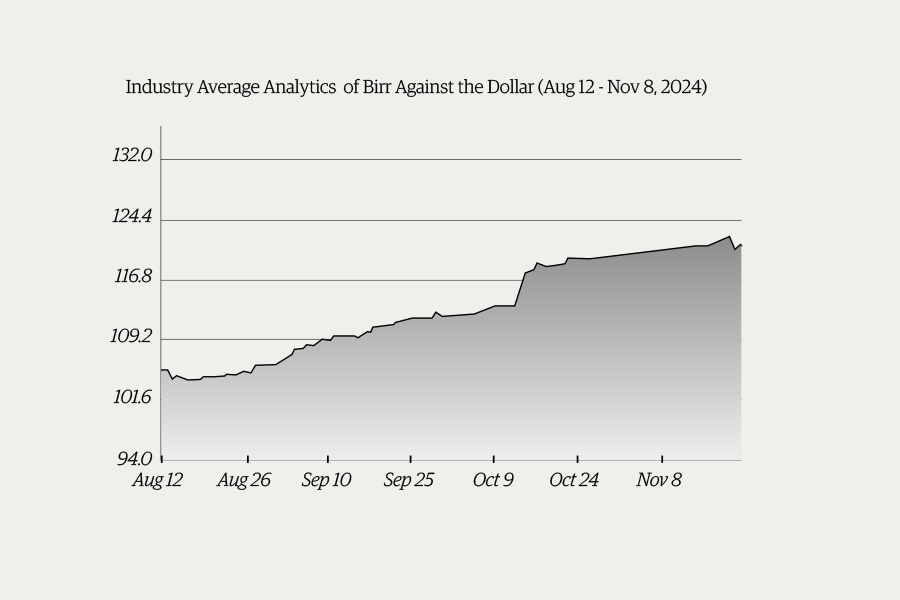
Money Market Watch | Nov 24,2024

Fortune News | Feb 22,2020

My Opinion | 132272 Views | Aug 14,2021

My Opinion | 128692 Views | Aug 21,2021

My Opinion | 126600 Views | Sep 10,2021

My Opinion | 124206 Views | Aug 07,2021





Dec 22 , 2024 . By TIZITA SHEWAFERAW
Charged with transforming colossal state-owned enterprises into modern and competitiv...

Aug 18 , 2024 . By AKSAH ITALO
Although predictable Yonas Zerihun's job in the ride-hailing service is not immune to...

Jul 28 , 2024 . By TIZITA SHEWAFERAW
Unhabitual, perhaps too many, Samuel Gebreyohannes, 38, used to occasionally enjoy a couple of beers at breakfast. However, he recently swit...

Jul 13 , 2024 . By AKSAH ITALO
Investors who rely on tractors, trucks, and field vehicles for commuting, transporting commodities, and f...
Jul 12 , 2025
Political leaders and their policy advisors often promise great leaps forward, yet th...

Jul 5 , 2025
Six years ago, Ethiopia was the darling of international liberal commentators. A year...

Jun 28 , 2025
Meseret Damtie, the assertive auditor general, has never been shy about naming names...
Jun 21 , 2025
A well-worn adage says, “Budget is not destiny, but it is direction.” Examining t...

Jul 13 , 2025 . By YITBAREK GETACHEW
The Addis Abeba City Revenue Bureau has introduced a new directive set to reshape how...

Jul 13 , 2025 . By BEZAWIT HULUAGER
Addis Abeba has approved a record 350 billion Br budget for the 2025/26 fiscal year,...

Jul 13 , 2025 . By RUTH BERHANU
The Addis Abeba Revenue Bureau has scrapped a value-added tax (VAT) on unprocessed ve...

Jul 13 , 2025 . By NAHOM AYELE
Federal lawmakers have finally brought closure to a protracted and contentious tax de...